SANTÉ

G4_ G quadruplexes


G4_ G quadruplexes
Structure des Acides Nucléiques, Télomères et Évolution
SANTE - Nucleic acid structures, Telomeres and Evolution
Telomeres are the nucleoprotein complexes that protect the ends of the linear chromosomes in eukaryotes. The SANTE team's research aims to contribute to the understanding of telomere regulation in normal and in pathological contexts as well as telomere evolution.
In addition to telomeres, the arrival in 2022 of a chemist in our team, has opened new research paths in the field of organic chemistry.
1 - Non-canonical nucleic acid structures and interactions with proteins
4 - Enhancing bioluminescent reporting systems
3 - Fishing G4-interacting proteins
• Carole Saintomé, PR Sorbonne Université I obtained my PhD in chemistry and biochemistry at the Pierre-Marie Curie University (now Sorbonne university) in Paris and joined the lab in 2011. I am interested in understanding the molecular mechanism that leads to telomere maintenance, in normal and in pathological contexts, and during evolution. My focus is on investigating the interactions between single-stranded DNA binding proteins and nucleic acids through a multidisciplinary approach at the intersection of chemistry and biochemistry
• Patrizia Alberti, MC Muséum I graduated in Physics at the University of Milan and obtained my PhD in Biophysics from the National Museum of Natural History in Paris. Following a post-doc in a biotechnology corporation, I joined this laboratory in 2005. My primary research focus is the characterization of non-canonical nucleic acid structures and how proteins interact with them. I am particularly interested in repeated sequences and in the evolution of telomeres, which are a fascinating illustration of the unity and diversity of Life.
• Jian-Sheng Sun, PR Muséum PhD in biophysics at the Pierre-Marie Curie University (now Sorbonne university) in Paris. I have carried out a dual career: in academic (CNRS/MNHN, 25 years) studying nucleic acid structures and functions, DNA damages and repairs, and in biotech (co-founder, CEO & chairman of DNA Therapeutics, 10 years) for developing a first-in-class anticancer drug made of a double-stranded DNA by interfering with DNA damage signaling mechanisms. Presently at the MNHN, I direct the department of "Adaptations of living organisms". I am also the scientific director of the "Bioinspire-Muséum", the co-pilot of the “Global approach of the Health” of the SOUND programs of the Alliance of Sorbonne Universities and scientific expert involved in the CBD-DSI negotiations during the French Presidency of European Union and the subsequent CBD-COP15 meetings.
• Anne-Laure Guieysse-Peugeot, PR Muséum Pr. Anne-Laure Guieysse-Peugeot obtained her PhD in Molecular Biology at the Muséum National d’Histoire Naturelle, in Paris. Throughout her career at the Museum, Anne Laure Guieysse-Peugeot has contributed to research on the mechanisms of regulation and gene expression. This work began with the study of the triple-helix structure of DNA and its involvement in the regulation of gene expression (1991-2000). The discovery of epigenetic mechanisms and their role in controlling gene expression led her to explore the role of DNA methylation in transcriptional regulation (2004-2014) followed by the roles of epigenetic mechanisms in telomere regulation during the aging process (2014-present).
• Laureline Roger, MC Muséum I obtained my PhD in molecular and cellular biology at Montpellier University of Science (France) and subsequently completed my postdoc at Cardiff School of Medicine (Wales UK) in Duncan Baird’s laboratory where I investigated the role of telomere dysfunction in carcinogenesis. During this time, I also collaborated with Kristin Ladell, where I used Single Telomere Length Analysis (STELA) to study the replicative history and differentiation of memory T-cells. I joined the National Museum of Natural History as a lecturer in 2014. My current research is focused on identifying the triggers and mechanisms of telomere fusions and how telomere dysfunction can contribute to cancer initiation.
• Yves Janin, DR CNRS Dr. Yves L. Janin obtained a Ph.D. in organic chemistry in 1993 under the guidance of Dr. Emile Bisagni at the Institut Curie. Following two years of postdoc at the ICSN, Gif/Yvette and a year in the Danish School of Pharmacy in Copenhagen, he then worked six years at the Institut Curie. After a sabbatical in the Vitry/Seine Aventis laboratories he then joined the Institut Pasteur until 2022. He is now working at the Muséum National d’Histoire Naturelle in Paris. His interest lies in medicinal chemistry, new chemical entities as well as in the design of original bioluminescence-based reporting systems.
• Gildas Mouta Cardoso, IE Muséum I joined the MNHN in 2003 to work in a proteomics platform. After obtaining a Master’s degree in chemistry in 2011, I joined Santé team as organic chemist to synthesize G-quadruplexes ligands. My principal research focus is the synthesis of small molecules for projects developed by the team. I am also involved in projects with others teams of the lab.
• Virginie Hossard, AI Inserm I obtained an advanced technician's certificate in Biochemistry in 2005 and an engineer assistant position in animal experimentation in 2007 within a team working on Biological Rhythms and Cancers at the Paul Brousse Hospital. I joined the laboratory in 2015 to work on several topics in cellular and molecular biology (G4 ligands, mitochondrial DNA, TERRA RNA, C-Circles) and more recently in biochemistry on the characterization of POT1 and RPA proteins involved in telomeric stability.
• Florian Gourmelon, CDD IE Muséum I obtained two master degrees in 2014 (biochemistry/cell biology) and 2015 (biochemistry/molecular biology) and I have joined in 2022 the Muséum National d’Histoire Naturelle in Paris as an engineer to produce, purify and characterize two proteins and their mutants (Replication Protein A / POT1-TPP1) involved in telomeric stability.
• Congcong LI, Doctorante Muséum
• Marianne Bechara, Doctorante MNHN After getting a PharmD degree at Saint Joseph Univeristy in Lebanon, I pursued a master’s degree in Genetics and Epigenetics at Sorbonne Univeristy. I joined the lab in January 2023 as an intern, and i am curently in my first year as a PhD student.
• Patrick Mailliet, Chercheur Bénévole Muséum I spent about 40 years in the pharmaceutical industry as team leader in Medicinal Chemistry and project manager in Oncology, with specific expertise in DNA-interacting compounds and kinases. From 2010, I worked at the MNHN on ligands of nucleic acid structures (mainly G-quadruplexes). I also advise the team on advances in Medicinal Chemistry and I teach in different masters. Apart from these activities, I am consultant for two small pharmaceutical companies, one in France and one in Italy.
Past members 2014 - 2023
Jean-François Riou, PR Muséum (2007-2023)
Anthony Bugaut, CR CNRS (2013-2020)
François Peurois, ATER (2018-2019)
Coralie Modeste, IE (2018-2019)
Jean Chatain, PhD student (2017-2020)
Gabriel Le Berre, PhD student (2016-2019)
Pauline Lejault, PhD student (2014-2017)
Frédéric Thiebaut, PhD student (2014-2017)
CURRENT RESEARCH
We have expertise in the characterisation of non-canonical DNA structures and the way in which single-stranded DNA-binding proteins interact with these structures. We are particularly interested in DNA sequences composed of tandem repeats of a short motif of 2-6 nucleotides, also known as STR (for short tandem repeats).
Our aim is to gain understanding into the plasticity and evolution of eukaryotes at the level of the molecular structures that ensure the protection of their chromosome ends.
In eukaryotes where telomeres are elongated by telomerase, the telomeric DNA strand running toward the 3' end is generally composed of repeats of a short motif ("telomeric motif") carrying consecutive guanines. Telomeric DNA associates (directly or indirectly) with a set of proteins specific of telomeres that are essential for telomere protection and regulation.
Our aim is to gain understanding into the proteins that, depending on their state (wild type, variant, isoform) contribute to telomere stability or instability.
In the past years, we have studied how the single-stranded DNA-binding proteins human RPA (hRPA) and human POT1-TPP1 (hPOT1-TPP1) deal with secondary structures (G-quadruplexes) formed by the telomeric DNA. In the framework of a collaborative research project funded by ANR (TeloRPA), we started a new research axis aimed at characterising variants hRPA and hPOT1 found in patients affected by telomeres biology disorders (TBDs).
One of our research projects is focused on the study of bioluminescence-based reporting systems which are made of a luciferin and a luciferase. As depicted below, coelenterazine is the substrate of a wide array of marine luciferases and its oxidative decarboxylation leads to the production of coelenteramide along with a blue photon, the most visible color undersea. Our previous work led to a synthetic process to prepare O-acetylated luciferins such as hikarazines-103 and 108 (doi: 10.1039/c9ob00459a; doi: 10.1002/chem.201904844).
In eukaryotes where telomeres are elongated by telomerase, the telomeric DNA strand running toward the 3' end ("G-strand") is generally composed of repeats of a short motif of 5–8 nucleotides ("telomeric motif") carrying 2, 3 or 4 consecutive guanines and, in a variety of eukaryotes, it ends with a 3' single-stranded overhang ("G-overhang"). The human telomeric motif is the hexamer GGGTTA. The GGGTTA motif has first been identified in human telomeres; it is conserved in vertebrates and is found in many other eukaryotes. The presence of consecutive guanines makes the telomeric G-strand prone to fold into G-quadruplexes (G4) (doi: 10.1093/nar/gkq1292).
Interfering with telomere regulation has a double interest: on the one hand, it is an approach to gain insight into telomere regulation; on the other hand, it is a potential approach against proliferation of tumour cells. The main results of our studies on this topic are outlined here below.
The figure here below synthetises our major results, detailed below.
One of the major challenges to explore G4 functions in cells and to design specific ligands is their intrinsic polymorphic structure. In the framework of a collaborative project funded by ANR (G4-TopIPro), we used chemically constrained G4 structures to stabilize a particular G4 DNA or RNA topology as baits to fish G4-interacting proteins. This allowed us to identify new G4-interacting proteins, such as the NELF complex involved in the RNA-Pol II pausing mechanism. NELF complex and RNA Pol II pausing favour DNA double-strand break induction following G4 stabilization by Pyridostatin (doi: 10.1038/s41598-021-92806-8).
Illustrations of the different themes of the team

Droplets formed by human RPA under fluorescent microscopy

G4 at telomeres

Walking along telomeres: G4, hairpins and SSB proteins

Interfering with telomeres: epigenetic and ligands
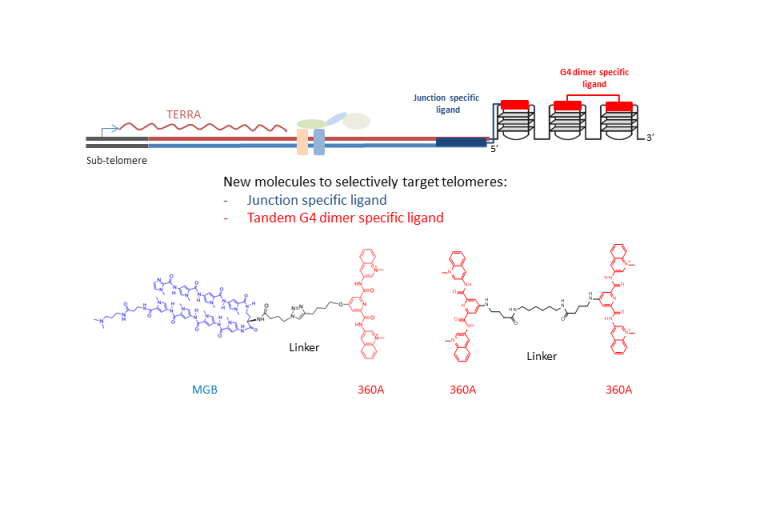
New molecules specifically targeting telomeres

Interfering with telomeres: AsiDNA

Protein network interacting with constrained G4 (Pipier et al. 2021)

RPA binding with multimeric telomeric G-quadruplexes studied by EMSA

Single TElomere Length Analysis (STELA)

Telomere crisis